The antioxidant glutathione (GSH)
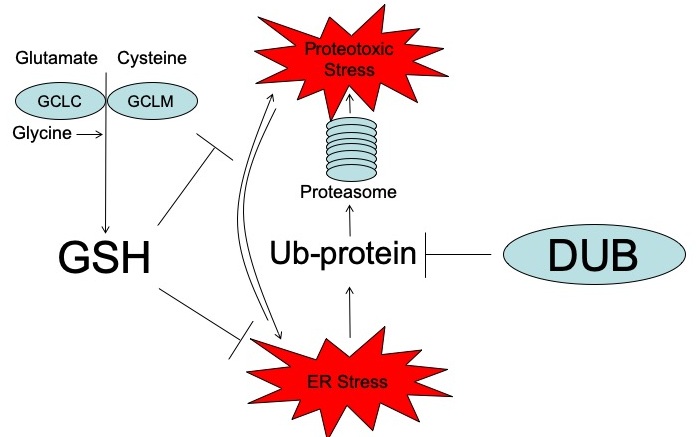
Discovered more than a century ago (De Rey-Pailhade, J., 1885), the most abundant antioxidant in the cell is glutathione (GSH). A tripeptide composed of glutamate, cysteine and glycine, the rate-limiting, non-redundant step in GSH synthesis is the condensation of glutamate and cysteine. This step is governed by a holoenzyme called glutamate-cysteine ligase which consists of a catalytic subunit (GCLC) and modifying subunit (GCLM). Acting as a cofactor, GSH works with enzymes to convert reactive oxygen species (ROS) into a benign species and quech oxidative stress. While GSH has been implicated in numerous cellular processes, many questions regarding its roles in the cell remain to be elucidated.
GSH and cancer initiation
There has been a long-standing belief that supplementation with antioxidants could prevent or treat cancer. However, results of large-scale clinical trials have proven the opposite: supplementation with antioxidants promote, rather than prevent, cancer. While these findings were indisputable, the mechanism behind these surprising results was lacking. A mechanism explaining the failure of antioxidants in preventing cancer was provided when it was demonstrated that the antioxidant GSH was required for cancer initiation (Harris, I.S., Cancer Cell, 2015). Since the systemic deletion of the catalytic enzyme in GSH synthesis (Gclc) in mice is embryonically lethal, a viable knockout of the GCLC modifying subunit (Gclm) was utilized with a ~75% reduction in GSH levels and without any gross phenotype. Crossing these mice to multiple spontaneous tumor models revealed that reduced GSH synthesis prevented the development of malignant cells. This result and subsequent mechanistic studies confirmed that cancer cells utilize antioxidants during tumor initiation to mitigate ROS levels.
GSH and protein homeostasis
While GSH synthesis was required for tumor initiation, it was found that reducing GSH levels in established tumors was not sufficient to inhibit tumor growth. In this context, additional pathways provided a backup system allowing cancer cells to survive in the absence of GSH. To interrogate these support systems, a high-throughput pharmacologic screening technique was developed to study vulnerabilities in cancer cells. Denoted Multifunctional Approach to Pharmacologic Screening (MAPS, below), enables users to determine the cellular response to perturbations of hundreds of molecular pathways. Using MAPS, it was discovered that deubiquitinases (DUBs) play an integral role in maintaining protein homeostasis in the absence of GSH. The combined inhibition of GSH and DUBs in cancer cells leads to aberrant proteotoxic stress and cell death (Harris, I.S., Cell Metabolism, 2019). This discovery, using unbiased, high-throughput approaches, demonstrated a key role for GSH in the regulation of protein homeostasis.
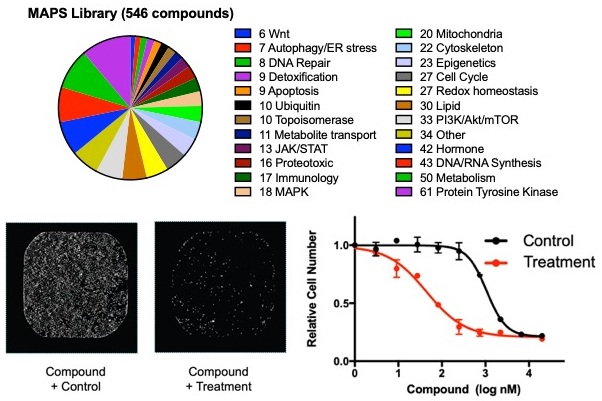
MAPS high-throughput technology
The Multifunctional Approach to Pharmacologic Screening (MAPS) is a high-throughput pharmacologic screening technique used by the Harris Lab to study vulnerabilities in cancer cells. This platform enables users to quickly and easily determine the cellular response to perturbations of hundreds of molecular pathways. The MAPS compound library is comprised of more than 500 inhibitors of cancer-related proteins as well as lesser studied metabolic enzymes. In contrast to traditional screening approaches, the MAPS library contain compounds arrayed across 10 dilution points, allowing for a robust understanding of the differential drug sensitivities. MAPS uses an image-based readout to determine drug sensitivities where cell nuclei are identified and counted. This technology has been used this powerful tool in >30 projects in collaboration with >15 different labs across the USA. These libraries have been shared openly with the research community and are available at the Harvard Medical School screening facility (https://bit.ly/2AvxtM4; https://bit.ly/2JkmyI0).
Genetically-engineered mouse models
For decades, genetically engineered mouse models (GEMMs) have been used to better understand cellular processes and their effects within a living animal. GEMMs can be used to study normal physiology, such as mammary gland developed (right), as well as pathophysiology, such as the MMTV-PyMT mouse model that spontaneously develops breast cancer. In the Harris lab, we use a variety of GEMMs to understand the roles of antioxidants in disease, with a focus on cancer development and progression.